Expert answer 100 2 ratings previous question next question get more help from chegg.
Why are vinylic hydrogens not acidic.
Other characteristics alkynes attached hydrogen have the formulas of h cc h or r cc h with r representing any alkyl group such as methyl ethyl etc.
It forms at least one double bond.
Vinylic c h bond is lower.
Vinylic carbon can have only two carbon as the maximum number.
Number of hydrogen atoms.
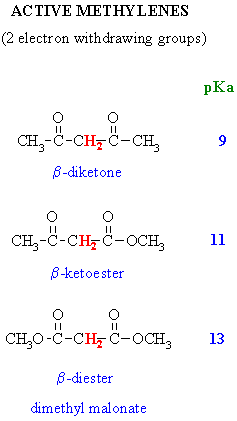
I m talking about the proton bonded to the carbonyl carbon not the alpha proton.
How many vinylic and allylic hydrogens does the following compound have.
Tautomerism essentially requires the presence of α hydrogen the hydrogen atom present generally adjacent to the functional group or the hydrogen atom present on the carbon which is adjacent to the carbon attached to the functional group.
Allylic carbon only forms a single bond.
Get 1 1 help now from expert chemistry tutors.
The first category of acids are the proton donors or brønsted lowry acids in the special case of aqueous solutions proton donors form the hydronium ion h 3 o and are known as arrhenius acids.
Vinylic chlorides and bromides constitute a diverse class of marine natural products.
Organohalogen compound organohalogen compound vinylic halides.
In general decreased hybridization increases acidity because there is greater s character in the orbital containing the lone pair so it is held closer to the nucleus.
Allylic carbon can have a maximum of 3 hydrogen atoms.
The gases are not toxic per se but can act to exclude oxygen in breathing zone during an incident.
Vinylic carbon can have either two double bonds in its sides or one double bond.
Or halide attached to the carbon.
Why is an aldehyde not acidic.
An acid is a molecule or ion capable of donating a proton hydrogen ion h a brønsted lowry acid or alternatively capable of forming a covalent bond with an electron pair a lewis acid.
This simple picture is complicated by the fact that movement of electrons in bonds particlarly pi bonds and delocalized systems.