Granite has little or no effect to acid rain whilst limestone is slowly eroded therefore granite lasts longer.
Why does acid rain affect granite more than limestone.
When sulfurous sulfuric and nitric acids in polluted air and rain react with the calcite in marble and limestone the calcite dissolves.
The presence of limestone and other calcium carbonate rock in lakes and streams helps to maintain a constant ph because the minerals react with the excess acid.
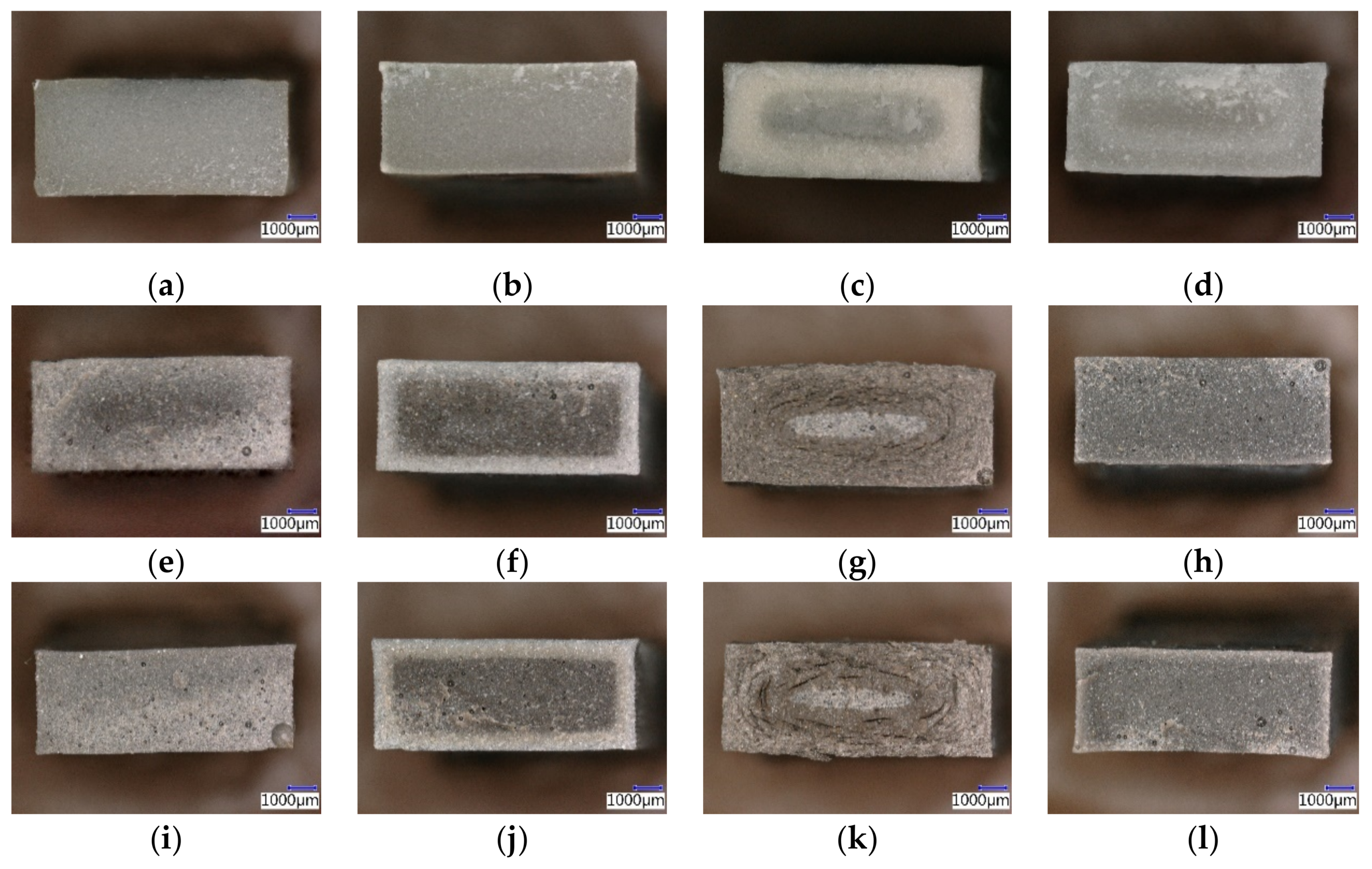
Stone surface material may be lost all over or only in spots that are more reactive.
Limestone is a rock that is composed of calcium carbonate caco3.
Acid rain is created by air pollutants.
When sulfurous sulfuric and nitric acids in polluted air react with the calcite in marble and limestone the calcite dissolves.
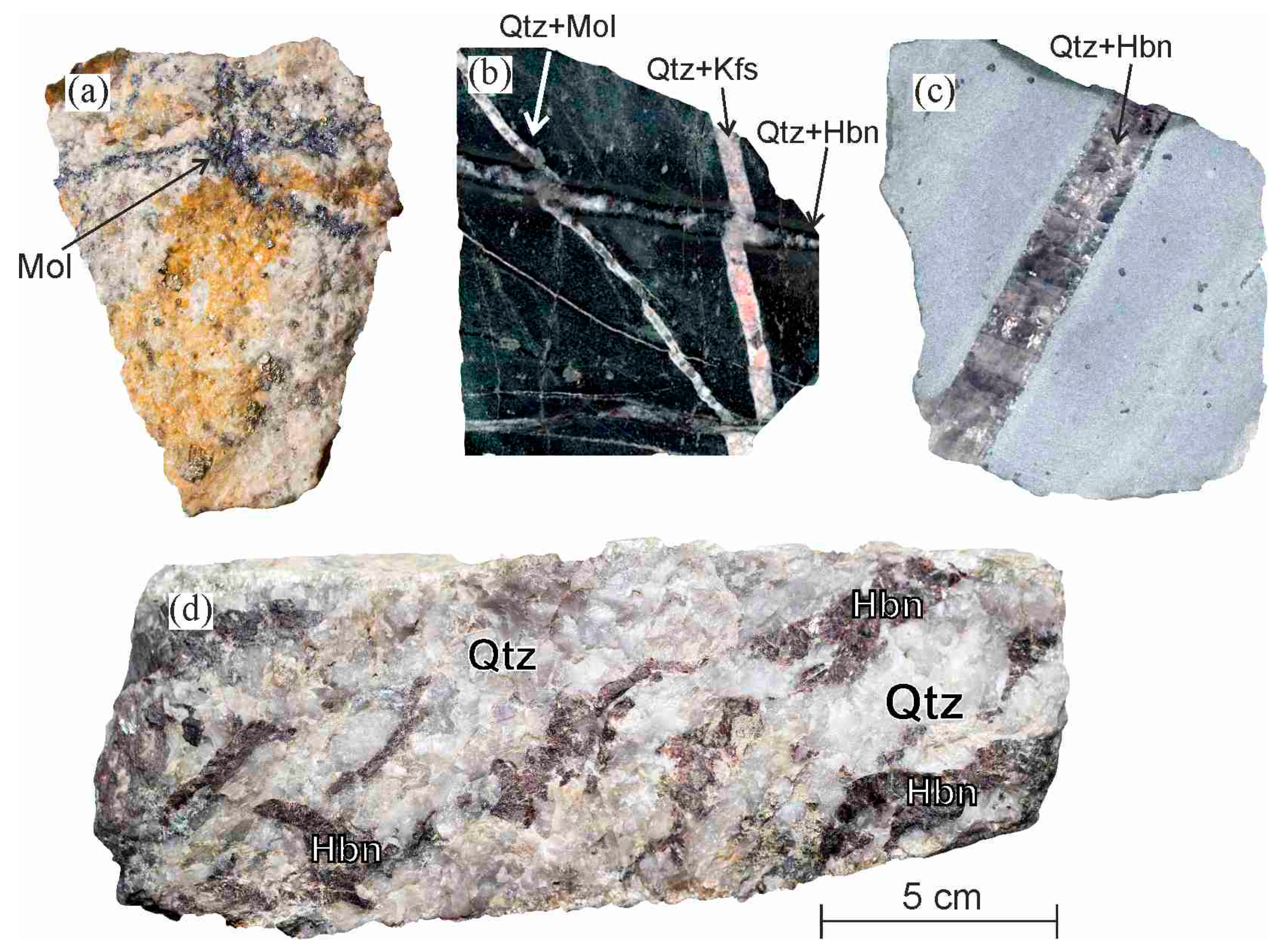
One of the most noticeable effects of acid rain is on limestone blocks that are part of a building or statue.
Because surface waters are in equilibrium with atmospheric carbon dioxide there is a constant concentration of carbonic acid h2co3 in the water.
In exposed areas of buildings and statues we see roughened surfaces removal of material and loss of carved details.
Limestone is mostly made up of the mineral calcium carbonate caco3.
Acid precipitation affects stone primarily in two ways.
How does acid precipitation affect marble and limestone buildings.
The chemicals fall to earth as acid rain.
This is not very soluble so rocks don t dissolve very quickly.
But if you add an acid you add hydrogen ions h which will react with the carbonate to form hydrogen carbonate hco3 ions which are very soluble in water and the limestone will dissolve.
Or if there is more acid two hydrogen ions will.
Acid rain is slowly destroying once resistant granite.
Sulphur dioxide a byproduct of industrial development and burning fossil fuels combines with nitrogen oxide an air pollutant created by car exhaust furnaces boilers and engines.
Another common reaction is the production of gypsum on the surface of the limestone that comes in contact with sulfuric acid.